Pipeline
Developing Differentiated Biologic Therapies
ADAXION’s technology is designed to enable the development of novel biologic therapies that overcome immunogenicity limitations.
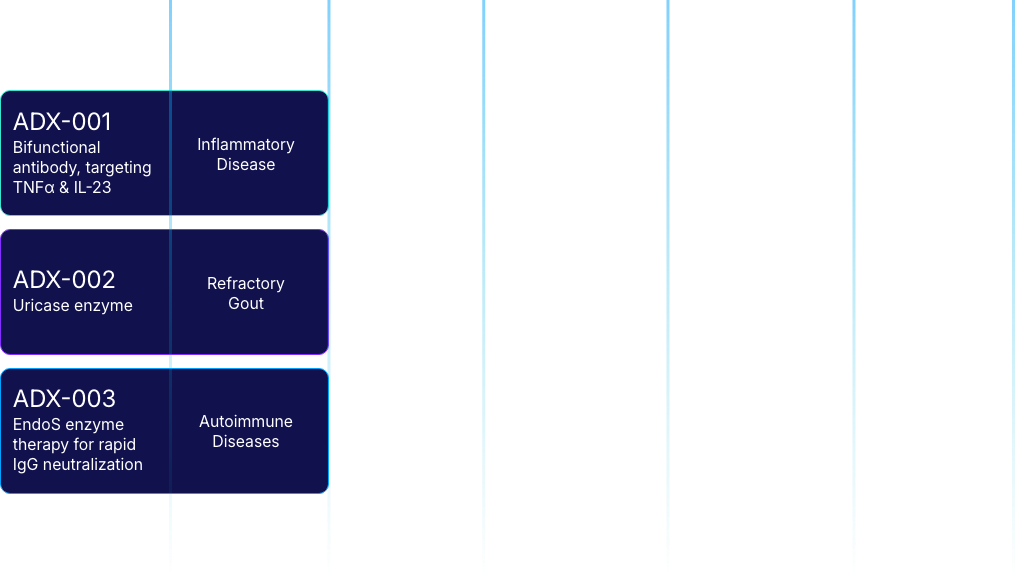
ADX-001: Bifunctional Antibody Targeting TNFα and IL-23 for Inflammatory Disease
- Reduced Immunogenicity: ADAx technology enables the development of a bispecific class that has historically exhibited prohibitive anti-drug antibodies (ADAs)
- Enhanced Efficacy: Dual TNFα and IL-23 targeting with clinical precedence shown to drive additive clinical remission rates compared to monotherapies
- Patient Convenience: Extended half-life design and amenable to subcutaneous dosing
- Streamlined Development Path: Bispecific approach overcomes development challenges associated with co-formulations
ADX-002: Enhanced Uricase Enzyme Therapy for Refractory Gout
- Reduced Immunogenicity: ADAx technology enables the development of a non-human enzyme that has historically exhibited prohibitive ADAs
- Enhanced Efficacy: Optimized for long-lasting uric acid reduction and tophi dissolution to improve patient outcomes
- Patient Convenience: Extended half-life design amenable to subcutaneous dosing potentially expanding access to patients who are unresponsive to existing therapies
ADX-003: EndoS IgG Deglycosylation Enzyme for IgG-driven Disease
- Novel Mechanism: Inhibits FcγR binding and complement activation of IgG while maintaining antibody levels
- Reduced Immunogenicity: ADAx technology is designed to enable the development of an otherwise immunogenic bacterial enzyme
- Enhanced Efficacy: Fast-acting mechanism eliminates IgG effector function within hours
- Patient Convenience: Catalytic activity enables potential for low dose treatment
